Connecting the healthcare community in the Americas
Source global medical products efficiently, connect lastingly
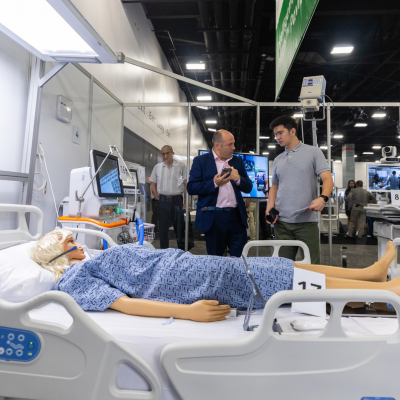
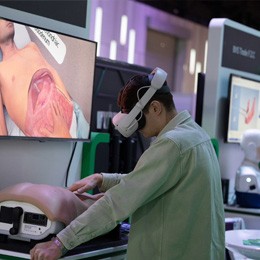
FIME is the must-see medical trade event in the Americas. It brings together innovative medical products and supplies, networking opportunities with regional and international key players in the industry, and the chance to elevate your business. Prepare to navigate the evolving landscape of medical product discovery.
About FIME?
Source medical products globally efficiently and establish lasting connections with over 16,000 healthcare attendees and a comprehensive showcase of 1,300 new and refurbished medical device and equipment manufacturers and suppliers. Renowned as the premier trade show in the Americas, FIME draws regional and international healthcare professionals who recognize the importance of learning, networking, and fostering business relationships.
What to expect in 2024?
With over $100 million worth of business generated on the show floor in 2023.
We are once again ready to welcome you back to business in June 2024 and offer you unlimited opportunities to grow your network and make new business connections.
Fully committed to delivering the best possible event to the healthcare community once again in 2024, the Florida International Medical Expo is all set to unite the global healthcare industry at the Miami Beach Convention Center.
See who is exhibiting
Industry Reports
Analyzing the Orthopedic Trauma Care Industry in the Americas

Download report
The Orthopedic Trauma Devices Market in the Americas is experiencing steady growth, fueled by innovative technologies like robotics, 3D printing, and computer-assisted navigation systems.
How AI Reshapes the Medical Imaging
Industry

Download report
In 2024, AI in the medical imaging market is valued at USD 5.86 billion and is projected to rise to over USD 20.40 billion by 2029. Despite sustained investment and an increasing array of certified products.
Breakthroughs in Laboratory Diagnostics for Chronic Diseases

Download report
Chronic diseases, or non-communicable diseases (NCDs), are persistent medical conditions that deteriorate over time. The illness lasts at leasta year, impedes daily activities and requires continuous medical attention.
Our commitment to sustainability
We know how sustainability is increasingly important
Running FIME gives us lots of opportunities to improve our impact environmentally, socially and economically in our region. We want to make the event more responsible and play a role in helping the market improve its own sustainability through connecting people with the networks and knowledge they want to help solve the big challenges in the healthcare sector.
2023 Post show report
FIME 2023 continues its legacy as the premier event for those engaged in the medical industry, providing an unparalleled platform to network, acquire knowledge, and delve into fresh global prospects.
Boasting a remarkable attendance of more than 13,000+ healthcare professionals and a diverse array of 1,158 exhibitors representing 116 countries, FIME 2023's vibrant physical presence facilitated the generation of an impressive $325.1 milion in business transactions throughout the event.
Testimonials
Join us in-person at FIME 2024
Register now
Register now for visit FIME 2024.
Book a stand
Apply for a stand at the 2024 Live, in-person event.